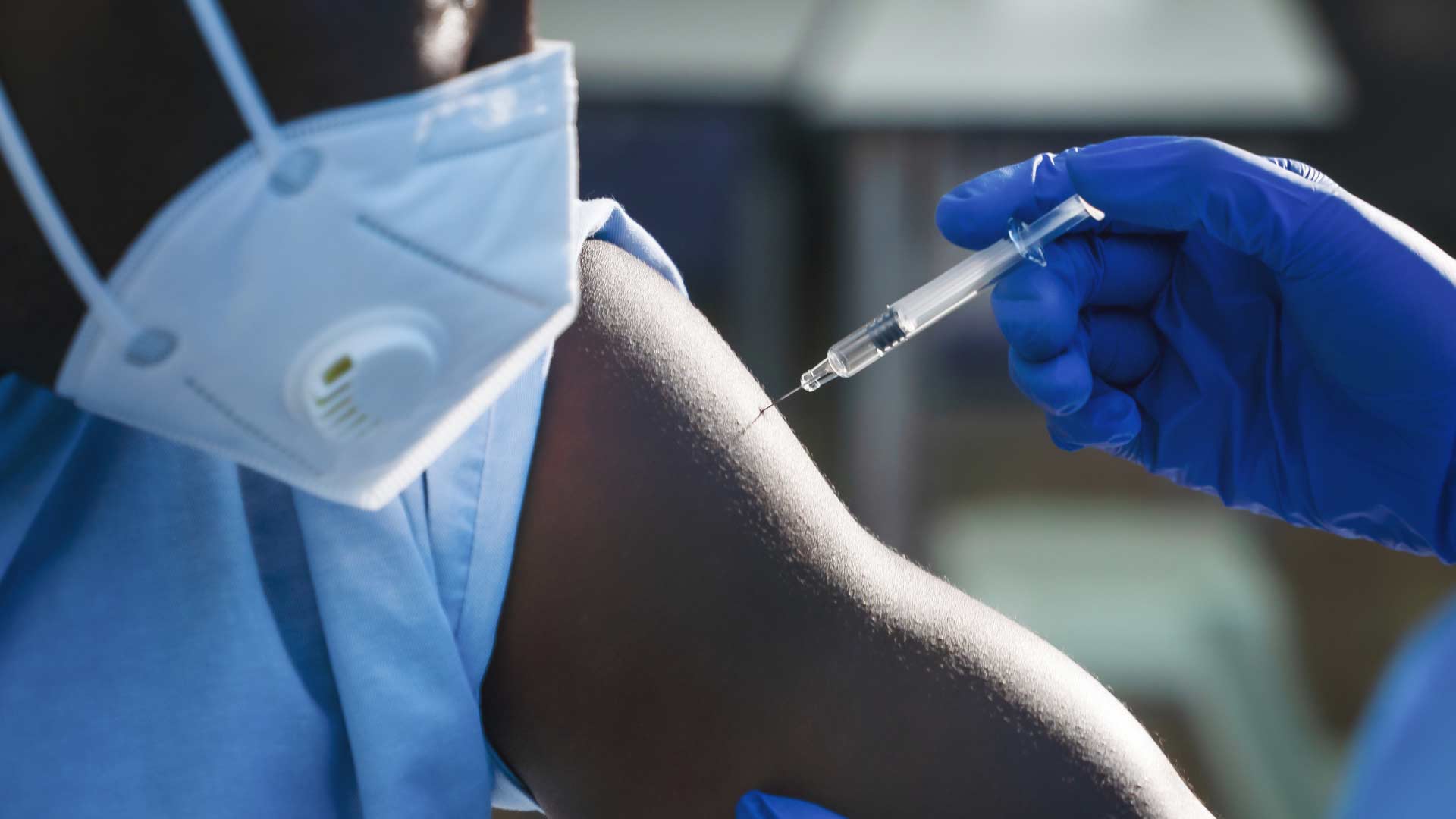
At least one health care worker in Juneau experienced a severe allergic reaction from getting the Pfizer COVID-19 vaccine on Dec. 15. The Alaska Dept. of Health and Social Services issued a statement about the incident on Dec. 16.

“A health care worker in Juneau who has no history of allergies had an anaphylactic reaction that included flushing and shortness of breath 10 minutes after receiving the vaccine at a clinic at Bartlett Hospital,” it states. “Symptoms were discovered during the 15-minute observation period recommended by the CDC.”
The health care worker took Benadryl after the symptoms began and when they did not resolve, the worker was admitted to the Emergency Department at Bartlett, the statement noted. The patient is in stable condition but is still in the hospital being monitored.
“We expected that a side effect like this could occur after reports of anaphylaxis were made in England after people there received the Pfizer-BioNTech COVID-19 vaccine,” said Alaska’s Chief Medical Officer Dr. Anne Zink. “All sites that are approved to provide vaccinations in Alaska must have medications on hand to deal with an allergic reaction and that was the case in Juneau.”
Clinics in Alaska will continue with the vaccines as planned, the state said with more clinics coming on board this week at hospitals around the state.
Similar anaphylactic reactions occurred in two British health care workers after they were given the Pfizer vaccine last week. Both recovered. These types of responses are due to allergic reactions. The Centers for Disease Control and Prevention warned that people who have had severe reactions to other vaccines should discuss the risks with their doctors.
News of the adverse reaction in a Alaska health care worker comes when the state is in the process of administering more than 35,000 Pfizer vaccines across the state as part of the national emergency rollout. The first Alaskans receiving the vaccine are front-line health care workers, long-term care facility residents and staff, EMS and fire personnel providing medical services, community health practitioners and people who perform vaccinations.
On Dec. 10, the U.S. Food and Drug Administration authorized Emergency Use Authorization (EUA) of the Pfizer vaccine. It is a mRNA vaccine, which has never been sanctioned for widespread use in humans. The EUA allows the FDA to make a vaccine available without going through the full review process. An FDA video explaining the EUA says it differs from normal protocols “because in some emergency situations, we just cannot wait for all the evidence needed for full FDA approval.”
ALASKA WATCHMAN DIRECT TO YOUR INBOX
On Dec. 10, the FDA released its analysis of the Pfizer vaccine, including known risks. Of the 44,000 people who participated in the clinical trials, this is the percentage who had adverse reactions: injection site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%). Severe reactions occurred in up to 4.6% of subjects.
The FDA analysis noted that there is “insufficient data to make conclusions about the safety of the vaccine in subpopulations such as children less than 16 years of age, pregnant and lactating individuals, and immunocompromised individuals.” It adds that once the vaccine is administered to a larger sample of the U.S. population, it may reveal additional “less frequent and/or more serious adverse events” which were not initially detected.






18 Comments
The Vaccine of the Beast to go along with your Mask of the Beast. Both are conditioning you for the ultimate Mark of the Beast.
No go Like Candy, are you reading ahead again?
Damn spellcheck. *Mongo
No sympathy WHATSOEVER – these are the people who will recommend the vaccine to others and shove needles into unsuspecting children’s arms. Vaccines are Russian roulette – you don’t KNOW if you will have a bad reaction – until you DO… and then it’s too late. And too often the side effects are worse than the ailment that it was supposed to protect you from. Tough shit!! Hope it wakes a few more of the “healthcare” workers up!
This does not necessarily mean the vaccine is unsafe for all. I got toradol in a hospital ER for a severe migraine and went into anaphylaxis. It is a possibility with any medication or vaccine. This is why, when you get vaccines, you are asked to stick around for 15 to 20 minutes. The reaction depends on the makeup of the person’s immune system.
Yeah right- so hey – take the risk guys – it’s WORTH it LOL. The flu vaccine hasn’t put a DENT in the ordinary ol flu in over 50 years and yet people think this one is going to kick covid into the ballpark. hey I’ve got a bridge to sell if anyone is interested?
Don’t need nazi box cars anymore the sheep will just line up and let you stick them. Keep lining up sheep have fun with your death needle. I’m not taking it!
A man with a BRAIN!!! You’re becoming part of an endangered species mate.
This article does nothing to give me any faith in a vaccine.
” $cience “
Soooo, we’re supposed to take a vaccine for a virus that kills 0.5% (that we know of, studies are saying that there are a LOT more people that have had covid but not been tested, so the death figure would be even smaller) but that vaccine has a 4.6% chance of “severe reaction”? Guess I’m kind of slow on the uptake, but does this make any sense?
You caught that too? Whew, there may be hope for intelligence after all
You got that one right : “makes no sense”.
Well I totally understand what everyone is saying about the vaccine. I refuse to take the vaccine and my friends are telling me I would rather die than take a shot that’s 99.97% effective??? I don’t understand how they got that data so quickly when you need years to study it’s effectiveness. I have lived thus far without catching Covid-19. Someone’s watching over me.
Hydroxchloroquine and other treatments are being used to help reduce the effects of the China plague. Those who have used them are ridiculed by the propaganda media, who are selectively indignant about the real dangers of vaccine.
And now hydroxchloroquine is a controlled substance but they have made the use of heroine legal.
China dealt with this virus…..without vaccine an won!they celebrate by putting facts behind the labels of drinks….an have opened a museum dedicated to how they dealt with it.
Something s wrong somewhere….I wont take it. They did nt need it. Why should we?
We need to discover why we cant get it right.
I am in two minds but like to know more before making a decision for or against.may we need total lock down with no movements to anywhere no shopping etc .to starve the virus to death.if it cant connect it cant feed and it will die out.hopefully.
They pulled hydroxychloroquine because to produce a vaccine this fast they had to be sure there were no other cures… follow Dr. Eric Nepute… he’s got some great info and is giving away zinc and vit D for the cost of shipping to help people stay healthy!
1 Trackback or Pingback