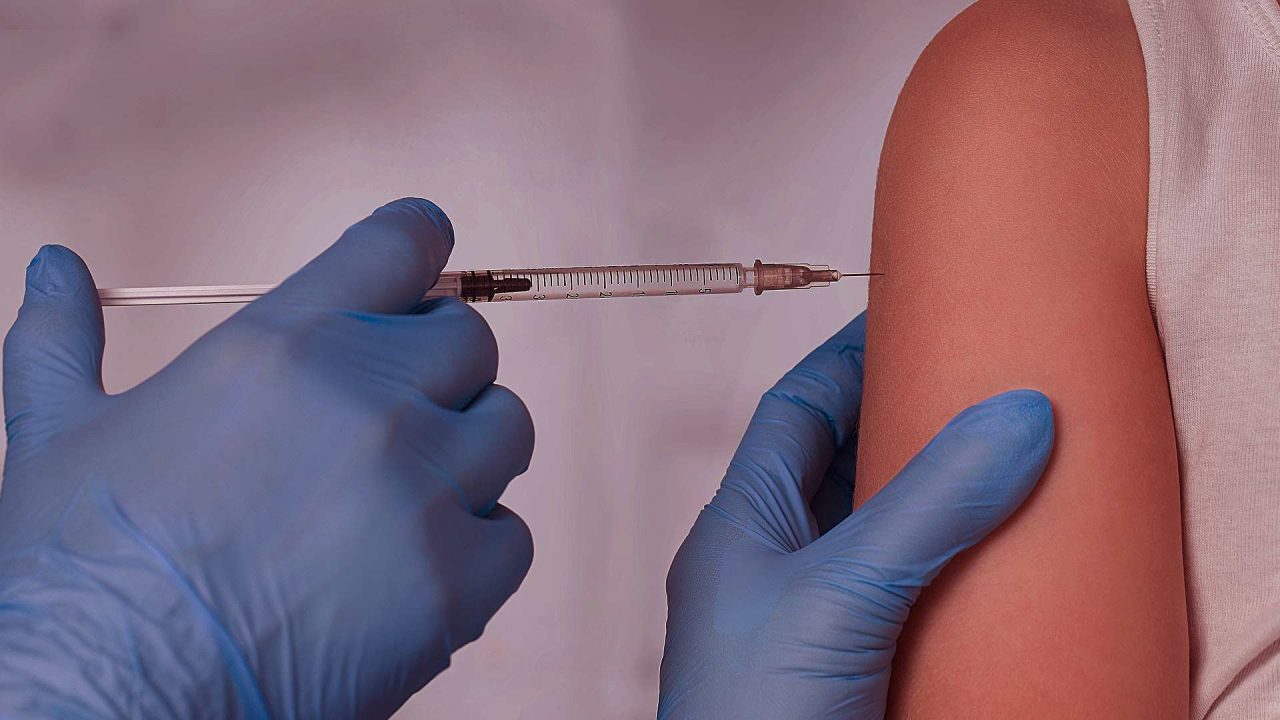
With Alaska just days away from receiving an expected 35,100 doses of Pfizer COVID-19 vaccinations, there are several considerations Alaskans should keep in mind when deciding whether to get the shot.
On Dec. 10, the U.S. Food and Drug Administration (FDA) met and voted 17-4 to approve Emergency Use Authorization (EUA) of the Pfizer vaccine. An EUA allows the FDA to make a vaccine available during a declared state of emergency without going through the full review process.
According to federal law, the FDA may allow unapproved medical products to be used in an “emergency.” An FDA video explaining the EUA notes that it differs from normal protocols “because in some emergency situations, we just cannot wait for all the evidence needed for full FDA approval.”
The video adds: “Instead the FDA evaluates the options very quickly using the evidence that is available, and carefully balances any known of potential risks of these unproven products with any known or potential benefits to the public of making them available during the emergency.”
This type of vaccine has never been licensed in the United States.
Vaccines are expected to arrive in Alaska in mid to late December with the Alaska COVID-19 Vaccine Task Force distributing the vaccine to health care providers shortly thereafter. The first Alaskans eligible to receive the vaccine will include front-line health care workers at highest risk for COVID-19 infection, long-term care facility residents and staff, EMS and fire personnel providing medical services, community health practitioners and people who perform vaccinations.
On Dec. 10, the FDA released its analysis of the Pfizer vaccine, including known risks. Of the 44,000 people who participated in the clinical trials, this is the percentage who had adverse reactions: injection site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%). Severe reactions occurred in up to 4.6% of subjects.
The FDA analysis noted that there is “insufficient data to make conclusions about the safety of the vaccine in subpopulations such as children less than 16 years of age, pregnant and lactating individuals, and immunocompromised individuals.”
ALASKA WATCHMAN DIRECT TO YOUR INBOX
The Pfizer vaccine, unlike all others approved by the FDA, is an mRNA vaccine. As opposed to traditional vaccines, which use dead, inactive or weakened versions of a virus, mRNA vaccines employ a new method that gives instructions to a person’s cells to make what is called a “spike protein” found on the surface of the virus that causes COVID-19. After the protein piece is made, the cell breaks down the instructions and gets rid of them. Next, the cell displays the protein piece on its surface, which causes an immune response and the production of antibodies against COVID-19.
This type of vaccine has never been licensed in the United States.
According to the FDA analysis, once the vaccine is administered to a larger sample of the U.S. population, it may reveal additional “less frequent and/or more serious adverse events” which were not initially detected in 44,000 people who participated in the clinical trials.
“Active and passive safety surveillance will continue during the post authorization period to detect new safety signals,” the FDA states.