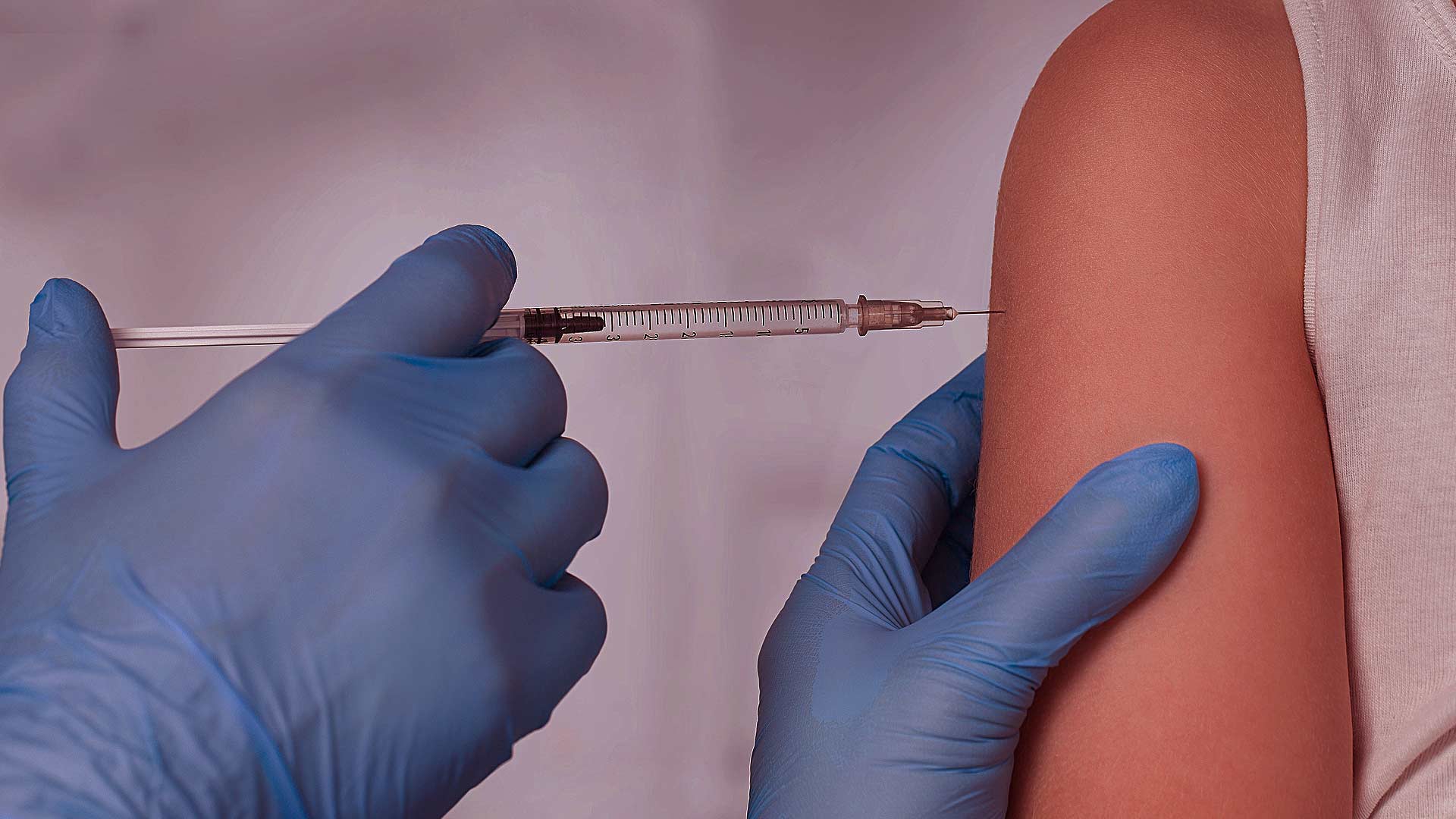
The first shipments of Pfizer COVID vaccines arrived on Sunday night via UPS from the Pfizer plant in Michigan. They quickly headed to health care facilities across Alaska with the first of two rounds of shots already administered to many residents.
Gov. Mike Dunleavy claims the vaccine will “help Alaskans put the worst behind us,” and suggested the shots would “begin the process of finally getting the upper hand of this pandemic and getting our lives back to normal.”
While the vaccine has not been granted full approval by the FDA, it did receive emergency use authorization (EUA) in 17-4 vote last week. An EUA allows the FDA to make a vaccine available during a declared state of emergency without going through the full review process.
The Pfizer vaccine is an experimental mRNA vaccine, which has never before been approved for widespread use in humans.
The initial vaccine supply is limited and medical providers and long term care residents. Eventually all Alaskans who wish to be vaccinated against COVID-19 will have an opportunity to do so. The governor has repeatedly said he has no plans to mandate vaccines for those who do not wish to take the fast-tracked vaccine.
The Pfizer vaccine requires two doses per person, separated by three weeks. The second dose is currently being held by Pfizer and will be shipped later. Everyone who receives the first dose will receive reminders to return for the second dose as the effectiveness of the vaccine depends on people receiving two doses three weeks apart.
On Dec. 10, the FDA released its analysis of the Pfizer vaccine, including known risks. Of the 44,000 people who participated in the clinical trials, this is the percentage who had adverse reactions: injection site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%). Severe reactions occurred in up to 4.6% of subjects.
ALASKA WATCHMAN DIRECT TO YOUR INBOX
The FDA analysis noted that there is “insufficient data to make conclusions about the safety of the vaccine in subpopulations such as children less than 16 years of age, pregnant and lactating individuals, and immunocompromised individuals.”
The Pfizer vaccine is an experimental mRNA vaccine, which has never before been approved for widespread use in humans. It requires extremely cold storage at temperatures ranging from -90 to -60° C (-130 to -76° F). To transport the vaccine, Pfizer designed shipping containers packed with dry ice that are capable of maintaining an ultra-cold temperature for up to 30 days as long as the dry ice is replenished upon receipt and every five days thereafter.
More vaccine is expected soon, both from Pfizer and from a second manufacturer, Moderna, whose emergency use authorization application is currently under review by the FDA. Pop-up drive-through sites and “points of dispensing” locations will be organized across Alaska, with the goal of making the vaccine easy to access for Alaskans who choose to get the shot.







1 Comment
I am finding conflicting “data” that says both the Pfizer and Moderna shots used aborted fetal tissue strains to test the vaccines on animals, but not directly in making the vaccines themselves. You should do your own research to verify, or disprove this. You are now forewarned. If it is true, you have to make a choice for or against life. No more fence straddling. It sure looks like the time is fast upon us to test our willingness to stand with Christ, or the world.